-
Browse Products
- Cable Equipments
- Cable Grips
- Cable Grips for domestic installation
- Cable Grips for fiber optical cables
- Cable Grips for overhead cabling
- Cable Grips for underground cabling
- productKatimex MULTIPLE CABLE GRIPS – DOUBLE EYE – GALVANIZED STEEL
- productKatimex CABLE GRIPS – DOUBLE EYE SPLIT – GALVANIZED STEEL
- productKatimex CABLE GRIPS – SINGLE LATERAL EYE – GALVANIZED STEEL
- productKatimex CABLE GRIPS – DOUBLE EYE – GALVANIZED STEEL
- productKatimex CABLE GRIPS – SINGLE EYE – GALVANIZED STEEL
- Cable support grips
- Suspension and Hose Securing Grips
- Swivels
- Wire and Cable Connector Grips
- Cable Pulling Systems
- Cable Grips
- Cables
- Fiber Optic Testing Equipment
- Copper / DSL testing
- Dispersion analysis
- Ethernet testing
- productEXFO FTB 5GPro – Complete, all-in-one 4G and 5G test solution
- productEXFO FTBx-88200NGE Power Blazer – 100G multiservice test module
- productEXFO MAX-800 Series – Ethernet and transport testing up to 100G
- productEXFO FTBx-8870/8880 Power Blazer – 10G multiservice test modules
- productEXFO FTBx-88260 – 1G to 100G network tester
- productEXFO FTBx-88460 – 400G test solution for the field
- productEXFO FTBx-88480 Series – compact, dual-port 400G testers for the field
- Fiber inspection
- productEXFO FIP-400B USB – Fiber inspection probe
- productEXFO MAX-FIP – Intelligent connector and fiber certifier
- productEXFO FIP-400B Wireless – Fiber inspection probe
- productEXFO FIPT-400-MF – Automated multifiber inspection solution
- productEXFO ConnectorMax MPO Link Test Solution – Polarity, continuity and connector testing
- productEXFO FIP-500 – Fiber inspection scope
- Light sources
- productEXFO FLS-140 – Visual fault locator
- productEXFO FiberBasix 50 – Handheld testers
- productEXFO FLS-240 Pocket Pal – Visual fault locator
- productEXFO FLS-300 – light source
- productEXFO FLS-600 – Light source
- productEXFO FLS-600 NS-1548 – Encircled flux compliant light source
- productEXFO PXM/LXM – MPO optical loss test set (OLTS)
- Live fiber detection
- Optical fiber multimeter
- Optical loss test kits
- productEXFO FOT-300 – Optical loss test set
- productEXFO FOT-600 – Optical loss test set
- productEXFO MaxTester 940/945 – fiber certifier OLTS
- productEXFO MaxTester 940/945 – Telco OLTS
- productEXFO FTBx-945 Fiber Certifier OLTS
- productEXFO FTBx-945 Telco OLTS
- productEXFO PXM/LXM – MPO optical loss test set (OLTS)
- OTDR and iOLM
- productEXFO FTB 5GPro – Complete, all-in-one 4G and 5G test solution
- productEXFO FTB-7400E – Metro/OTDR
- productEXFO FTB-7600E – Ultra-long-haul OTDR
- productEXFO FTB-LTC/PSB/SPSB – Launch test cable/pulse suppressor box
- productEXFO MaxTester 715B – last-mile OTDR
- productEXFO SPSB-EF – Encircled Flux Launch Mode Conditioner
- productEXFO FTBx-720C – LAN/WAN access OTDR
- productEXFO FTBx-730C – PON FTTx/MDU OTDR
- productEXFO FTBx-735C – metro/PON FTTx/MDU OTDR
- productEXFO 740C xWDM OTDR Series
- productEXFO FTBx-750C – metro/longhaul OTDR
- productEXFO MaxTester 720C – access OTDR
- productEXFO MaxTester 730C – PON/metro OTDR
- productEXFO TK-SWITCH MPO Test Kit – iOLM-based automated MPO cable characterization solution
- productEXFO FastReporter – Data post-processing software
- productEXFO Optical Wave Expert – DWDM Channel Checker and OTDR
- productEXFO MaxTester 715D – last-mile OTDR
- productEXFO MaxTester 730D – PON/metro OTDR
- productEXFO FTBx-730D – PON FTTx/MDU OTDR
- productEXFO iOLM – intelligent Optical Link Mapper
- productEXFO D-series OTDR line
- Power meters
- productEXFO PPM-350C – PON Power Meter
- productEXFO MPC-100 – optical power checker
- productEXFO PPM-350D – Next-gen PON power meter
- productEXFO Optical Power Expert – Connected optical power meter
- productEXFO PPM1 – Service activation PON power meter
- productEXFO FiberBasix 50 – Handheld testers
- productEXFO PXM/LXM – MPO optical loss test set (OLTS)
- RF testing
- Spectral testing
- productEXFO FOT-5200 – CWDM Channel Power Analyzer
- productEXFO FTBx-5245/5255 – Optical spectrum analyzers for the field
- productEXFO FOT-5205 – DWDM channel checker
- productEXFO FTBx-5235 – Optical spectrum analyzer
- productEXFO MAX-5205 – Simple DWDM channel checker
- productEXFO FTBx-5205 – Simple DWDM channel checker
- productEXFO Optical Wave Expert – DWDM Channel Checker and OTDR
- Weather and environmental measurement solution
- Automated weather stations
- Sounding systems & radiosondes
- Weather & Environmental Sensors
- Atmospheric Profiling
- Cloud height measurement
- Precipitation Sensors
- productVaisala Radiation Shield Series DTR500
- productVaisala Rain Gauge RG13
- productVaisala Rain Detector DRD11A
- productVaisala Rain Gauge QMR101 & QMR102
- productVaisala Visibility & Present Weather Sensors FS11 & FS11P
- productVaisala Present Weather Detectors and Visibility Sensors PWD Series
- productVaisala Forward Scatter Sensor FD70
- productVaisala Weather Transmitter WXT530 Series
- Pressure Sensors
- Road and Runway Surface Condition Sensors
- Temperature and Humidity Sensors
- Visibility & Present Weather Sensors
- Weather Radars
- Wind Sensors
- productVaisala Wind Set WA15 & WA25
- productVaisala Wind Sensor WM30
- productVaisala WINDCAP Ultrasonic Wind Sensor WMT700
- productVaisala Weather Transmitter WXT530 Series
- Power (electric) test solutions
- Cable fault and test system vans
- Cable fault locating equipment
- productMegger HPA 100/110/120 AC and 130 DC
- productMegger Teleflex VX
- productMegger Teleflex® SX-1
- productMegger TDR500/3
- productMegger TDR2050
- productMegger TDR2000/3 and TDR2010
- productMegger TDR1000/3P
- productMegger STX 40
- productMegger SFX5-1000 and SFX8-1000
- productMegger SFX32
- productMegger MFM10
- productMegger Ferrolux Rx
- productMegger ESG NT
- productMegger digiPHONE+2
- Cable testing and diagnostics
- productMegger UHF PD Detector
- productMegger VLF Sine Wave 62 kV
- productMegger VLF Sine Wave 45 kV
- productMegger VLF Sine Wave 34 kV
- productMegger VLF CR-60HP and VLF CR-80
- productMegger VLF CR-28, VLF CR-40 and VLF CR-60
- productMegger TDS NT
- productMegger TDM45
- productMegger Tan delta attachment
- productMegger T26/1A 200 kV, T26/1B 400 kV and T26/1C 800 kV
- productMegger PDS 62-SIN
- productMegger PDS 60-v2
- productMegger PD Scan
- productMegger HV Tester 25
- productMegger HV test set 50 kV, HV test set 80 kV and HV test set 110 kV
- productMegger HV DAC series
- productMegger HPG50-H, HPG70-H, HPG80-H and HPG110-H
- productMegger HPG50-D, HPG70-D and HPG80-D
- productMegger EasyTest 20 kV
- Circuit breaker testing
- productMegger DLRO100E, DLRO100X and DLRO100H
- productMegger TRAX
- productMegger VIDAR
- productMegger TM1800
- productMegger TM1700
- productMegger SPI225
- productMegger SDRM202
- productMegger SDRM201
- productMegger OTS80PB and OTS60PB
- productMegger OTS100AF, OTS80AF and OTS60AF
- productMegger OTD Oil Tan Delta
- productMegger ODEN AT
- productMegger MOM690
- productMegger MOM600A
- productMegger MOM200A
- productMegger MOM2
- productMegger MJÖLNER600
- productMegger MJÖLNER200
- productMegger KF875 and KF-LAB MkII
- productMegger INGVAR
- productMegger EGIL
- productMegger DLRO600
- productMegger DLRO200
- productMegger CSU600A and CSU600AT
- productMegger CABA Win
- productMegger BALTO Modular
- productMegger BALTO Compact
- Battery testing equipment
- Earth testing
- Insulation resistance testing < 1 kV
- Low resistance ohmmeters
- Multifunction installation testers
- Multimeters and clampmeters
- productMegger PVM210
- productMegger PVK330
- productMegger PVK320
- productMegger MET1000
- productMegger DPM1000
- productMegger DCM340
- productMegger DCM330
- productMegger DCM310 and DCM320
- productMegger DCM305E
- productMegger DCM300E
- productMegger DCM1500S
- productMegger DCM1500
- productMegger AVO®830 and 835
- productMegger AVO®410
- productMegger AVO®210
- Portable appliance testing (PATs)
- Time domain reflectometers
- Voltage detectors
- Current and voltage transformer testing
- AC insulation testing
- productMegger T22/13B
- productMegger VAX020
- productMegger T22/1
- productMegger IDAX300 and IDAX350
- productMegger HPG 35/58/78 AC
- productMegger HPA 100/110/120 AC and 130 DC
- productMegger CDAX 605
- productMegger DELTA4000 Series
- productMegger HPG50-H, HPG70-H, HPG80-H and HPG110-H
- productMegger HPG50-D, HPG70-D and HPG80-D
- DC diagnostic insulation testing
- DC overvoltage or withstand testing
- VLF Insulation testing
- Motor and generator testing
- Relay and protection testing
- productMegger SMRT46 MULTI-PHASE RELAY TESTER
- productMegger SMRT43D
- productMegger RTMS
- productMegger SVERKER750/780
- productMegger SVERKER650
- productMegger SMRT1
- productMegger SVERKER900
- productMegger STVI
- productMegger SMRT46D
- productMegger SMRT43
- productMegger SMRT410D
- productMegger SMRT410
- productMegger SMRT33
- productMegger MLLF
- productMegger FREJA549
- productMegger FREJA546
- productMegger FREJA543
- Primary injection test systems
- Power quality (Megger)
- Power transformer testing
- productMegger MWA300 and MWA330A
- productMegger TTR550005B
- productMegger TTR20-1
- productMegger TTR100-1
- productMegger TTRU3
- productMegger MTO300 and MTO330
- productMegger MTO250
- productMegger MTO210
- productMegger Resonating inductor
- productMegger FRAX99, FRAX101 and FRAX150
- productMegger Power transformer test van
- productMegger VAX020
- productMegger IDAX300 and IDAX350
- productMegger CDAX 605
- productMegger DELTA4000 Series
- productMegger TRAX
- productMegger OTS80PB and OTS60PB
- productMegger OTS100AF, OTS80AF and OTS60AF
- productMegger OTD Oil Tan Delta
- productMegger KF875 and KF-LAB MkII
- Keysight
- Power Meters & Power Sensors
- productKeysight V3500A Handheld RF Power Meter
- productKeysight N1913PM5B VDI Erickson PM5B MM–Wave Power Meter with W-Band Sensor, Optional Tapers to 1.5 THz
- 8480 Series Power Sensors
- E-Series CW Power Sensors
- E9300 Average Power Sensors
- E9320 Peak and Average Power Sensors
- productKeysight E9326A Peak and Average Power Sensor, 50 MHz – 18 GHz, 1.5MHz Bandwidth
- productKeysight E9325A Peak and Average Power Sensor, 50 MHz – 18 GHz, 300kHz Bandwidth
- productKeysight E9323A Peak and Average Power Sensor, 50 MHz – 6 GHz, 5MHz Bandwidth
- productKeysight E9322A Peak and Average Power Sensor, 50 MHz – 6 GHz, 1.5MHz Bandwidth
- productKeysight E9321A Peak and Average Power Sensor, 50 MHz – 6 GHz, 300kHz Bandwidth
- EPM and EPM-P Series Power Meter
- N8480 Series Power Sensors
- P-Series Power Meter
- P-Series Wideband Power Sensors
- USB & LAN Power Sensors
- productKeysight U8485A DC/10 MHz – 33 GHz USB Thermocouple Power Sensor
- productKeysight U2064XA 10 MHz to 40 GHz USB Wide Dynamic Range Peak and Average Power Sensor
- productKeysight U2002H USB Power Sensor, 50 MHz to 24 GHz
- productKeysight U2000B 10 MHz – 18 GHz USB Power Sensor
- productKeysight L2063XA 10 MHz to 33 GHz LAN Wide Dynamic Range Average and Peak Power Sensor
- Waveguide Power Sensors
- Frequency Counter Products
- Generators, Sources + Power
- PXI Signal Generators
- productKeysight M9421A VXT PXIe Vector Transceiver
- productKeysight M9415A VXT PXI Vector Transceiver, 380 MHz to 6/8/12 GHz
- productKeysight M9411A VXT PXI Vector Transceiver
- productKeysight M9410A VXT PXI Vector Transceiver, 300/600/1200 MHz Bandwidth
- productKeysight M9381A PXIe Vector Signal Generator
- productKeysight M9380A PXIe Continuous Wave (CW) Source
- productKeysight M9383A PXI Microwave Signal Generator, 1 MHz to 44 GHz
- Signal Generators & Signal Sources
- productKeysight N5192A UXG X-Series Vector Adapter, Modified Version, 50 MHz to 20 GHz
- productKeysight EXG Signal Generators
- productKeysight MXG Signal Generators
- productKeysight X‑Series Signal Generators – MXG,EXG, and CXG
- productKeysight PSG Signal Generators
- productKeysight X-Series Agile Signal Generators – UXG
- productKeysight VXG Microwave Signal Generators
- PSG Signal Generators
- VXG Microwave Signal Generators
- X-Series Agile Signal Generators – UXG
- productKeysight N5194A UXG X-Series Agile Vector Adapter, 50 MHz to 40 GHz
- productKeysight N5193A UXG X-Series Agile Signal Generator, 10 MHz to 40 GHz
- productKeysight N5192A UXG X-Series Vector Adapter, Modified Version, 50 MHz to 20 GHz
- productKeysight N5191A UXG X-Series Agile Signal Generator, Modified Version
- X‑Series Signal Generators – MXG,EXG, and CXG
- productKeysight N5166B CXG RF Vector Signal Generator, 9 kHz to 6 GHz
- productKeysight MXG Signal Generators
- Waveform/Function Generators
- Arbitrary Waveform Generators
- M8100 Series Arbitrary Waveform Generators
- M9300 Series Arbitrary Waveform Generators
- PXI Arbitrary Waveform Generators
- productKeysight M3302A PXIe AWG and Digitizer Combination 500 MSa/s, 16 bit, and 500 MSa/s, 14 bit
- productKeysight M3202A PXIe Arbitrary Waveform Generator, 1 GSa/s, 14 bit, 400 MHz
- productKeysight M3201A PXIe Arbitrary Waveform Generator, 500 MSa/s, 16 bit, 200 MHz
- productKeysight M3300A PXIe AWG and Digitizer Combination, 500 MSa/s, 16 bit and 100 MSa/s, 14 bit
- USB Arbitrary Waveform Generators
- productKeysight U2331A 64-Channel 1 MSa/s USB Modular Multifunction Data Acquisition
- productKeysight U2353A 16-Channel 500 kSa/s USB Modular Multifunction Data Acquisition
- productKeysight U2761A USB Modular Function Generator
- productKeysight U2356A 64-Channel 500 kSa/s USB Modular Multifunction Data Acquisition
- productKeysight U2352A 16-Channel 250 kSa/s USB Modular Multifunction Data Acquisition
- EDU33210A Series Smart Bench Essentials Waveform and Function Generators
- Trueform Series Waveform/Function Generators
- productKeysight 33622A Waveform Generator, 120 MHz, 2 Channel
- productKeysight 33621A Waveform Generator, 120 MHz, 1 Channel
- productKeysight 33612A Waveform Generator, 80 MHz, 2 Channel
- productKeysight 33611A Waveform Generator, 80 MHz, 1 Channel
- productKeysight 33522B Waveform Generator, 30 MHz, 2-Channel with Arb
- productKeysight 33521B Waveform Generator, 30 MHz, 1-Channel with Arb
- productKeysight 33520B Waveform Generator, 30 MHz, 2-Channel
- productKeysight 33519B Waveform Generator, 30 MHz, 1-Channel
- productKeysight 33512B Waveform Generator, 20 MHz, 2-Channel with Arb
- productKeysight 33511B Waveform Generator, 20 MHz, 1-Channel, with Arb
- productKeysight 33510B Waveform Generator, 20 MHz, 2-Channel
- productKeysight 33509B Waveform Generator, 20 MHz, 1-Channel
- Arbitrary Waveform Generators
- PXI Signal Generators
- LCR Meters and Impedance Measurement Products
- Meters
- Digital Multi meters (DMM)
- Benchtop
- Handheld Digital Multimeters
- Specialty Digital Multimeters
- productKeysight U3606B Multimeter/DC Power Supply
- productKeysight 34450A Digital Multimeter, 5.5 Digit, OLED Display
- productKeysight 34420A Nano-Volt/Micro-Ohm Meter, 7.5 Digit
- productKeysight EDU34450A Smart Bench Essentials Digital Multimeter
- productKeysight 3458A Digital Multimeter, 8.5 Digit
- U1450A/60A Series Handheld Insulation Resistance Tester
- productKeysight U1461A Insulation Multimeter, OLED display, 50 V to 1 kV
- productKeysight U1453A Insulation Resistance Tester, OLED display, 50 V to 1 kV
- productKeysight U1452AT Telecommunications Insulation Resistance Tester, 50 V to 100 V
- productKeysight U1452A Insulation Resistance Tester, 50 V to 1000 V
- productKeysight U1451A Insulation Resistance Tester, 250 V to 1 kV
- Digital Multi meters (DMM)
- Oscilliscopes & Analyzers
- Spectrum Analyzers (Signal Analyzers)
- productKeysight N9032B PXA Signal Analyzer 2 Hz to 265 GHz
- productKeysight N9000B CXA Signal Analyzer 9 kHz to 265 GHz
- Basic Spectrum Analyzers
- FieldFox Handheld RF and Microwave Analyzers
- productKeysight N9914B FieldFox Handheld RF Analyzer, 6.5 GHz
- productKeysight N9913B FieldFox Handheld Microwave Analyzer, 4 GHz
- productKeysight N9963B FieldFox Handheld Microwave Signal Analyzer, 54 GHz
- productKeysight N9962B FieldFox Handheld Microwave Signal Analyzer, 50 GHz
- productKeysight N9961B FieldFox Handheld Microwave Signal Analyzer, 44 GHz
- productKeysight N9960B FieldFox Handheld Microwave Signal Analyzer, 32 GHz
- productKeysight N9953B FieldFox Handheld Microwave Analyzer, 54 GHz
- productKeysight N9952B FieldFox Handheld Microwave Analyzer, 50 GHz
- productKeysight N9950B FieldFox Handheld Microwave Analyzer, 32 GHz
- productKeysight N9962A FieldFox Handheld Microwave Spectrum Analyzer, 50 GHz
- productKeysight N9961A FieldFox Handheld Microwave Spectrum Analyzer, 44 GHz
- productKeysihgt N9960A FieldFox Handheld Microwave Spectrum Analyzer, 32 GHz
- productKeysight N9951B FieldFox Handheld Microwave Analyzer, 44 GHz
- productKeysight N9951A FieldFox Handheld Microwave Analyzer, 44 GHz
- productKeysight N9950A FieldFox Handheld Microwave Analyzer, 32 GHz
- productKeysight N9938B FieldFox Handheld Microwave Spectrum Analyzer, 26.5 GHz
- productKeysight N9937B FieldFox Handheld Microwave Spectrum Analyzer, 18 GHz
- productKeysight N9936B FieldFox Handheld Microwave Spectrum Analyzer, 14 GHz
- productKeysight N9935B FieldFox Handheld Microwave Spectrum Analyzer, 9 GHz
- productKeysight N9934B FieldFox Handheld Microwave Spectrum Analyzer, 6.5 GHz
- productKeysight N9933B FieldFox Handheld Microwave Spectrum Analyzer, 4 GHz
- productKeysight N9926A FieldFox Handheld Microwave Vector Network Analyzer, 14 GHz
- productKeysight N9925A FieldFox Handheld Microwave Vector Network Analyzer, 9 GHz
- productKeysight N9918B FieldFox Handheld Microwave Analyzer, 26.5 GHz
- productKeysight N9918A FieldFox Handheld Microwave Analyzer, 26.5 GHz
- productKeysight N9917B FieldFox Handheld Microwave Analyzer, 18 GHz
- productKeysight N9917A FieldFox Handheld Microwave Analyzer, 18 GHz
- productKeysight N9916B FieldFox Handheld Microwave Analyzer, 14 GHz
- productKeysight N9916A FieldFox Handheld Microwave Analyzer, 14 GHz
- productKeysight N9915A FieldFox Handheld Microwave Analyzer, 9 GHz
- productKeysight N9914A FieldFox Handheld RF Analyzer, 6.5 GHz
- productKeysight N9913A FieldFox Handheld Microwave Analyzer, 4 GHz
- X-Series Signal Analyzers
- productKeysight N9042B UXA Signal Analyzer, 2 Hz to 50 GHz
- productKeysight N9040B UXA Signal Analyzer, 2 Hz to 50 GHz
- productKeysight N9032B PXA Signal Analyzer, 2 Hz to 26.5 GHz
- productKeysight N9030B PXA Signal Analyzer, 2 Hz to 50 GHz
- productKeysight N9021B MXA Signal Analyzer, 10 Hz to 50 GHz
- productKeysight N9020B MXA Signal Analyzer, 10 Hz to 50 GHz
- productKeysight N9010B EXA Signal Analyzer, 10 Hz to 44 GHz
- productKeysight N9000B CXA Signal Analyzer, 9 kHz to 26.5 GHz
- productKeysight N9041B UXA Signal Analyzer 2 Hz to 110 GHz
- Power Meters & Power Sensors
- IT & Telecom test solutions
- Installation and Test
- Fiber Optic Testing, Inspection, and Cleaning
- productFluke Network FI-3000 FiberInspector™ Ultra Camera
- productFluke networks VisiFault™ Visual Fault Locator – Cable Continuity Tester
- productFluke Fiber QuickMap™
- productFluke FI-7000 FiberInspector™ Pro
- productFluke Network SimpliFiber Pro Optical Power Meter and Fiber Test Kits
- productFluke Network MultiFiber Pro Optical Power Meter and Fiber Test Kits
- productFluke Network Fiber Optic Cleaning Kits
- Network cable testers
- Fiber Optic Testing, Inspection, and Cleaning
- Cabling certification
- Fusion Splicers, Fiber Strippers, Fiber Cleavers and Fiber Identifiers
- productFujikura Ribbon Fiber Stripper RS02/03
- productFujikura Optical Fiber Identifier FID-30R/31R/32R
- productFujikura Optical Fiber Cleaver CT08
- productFujikura Optical Fiber Cleaver CT50
- productFujikura Mass Fusion Splicer 41R Kit
- productFujikura Single Fiber Stripper SS01/03
- productFujikura Clad Alignment Fusion Splicer 41S+ Kit
- productFujikura Mass Fusion Splicer 90R Kit series
- productFujikura Core Alignment Fusion Splicer 90S+ Kit
- Installation and Test
- Fluke Calibration
- Temperature Calibration
- Electrical Calibration
- Bench Multimeters
- Electrical Calibrators
- product5080A High Compliance MultiProduct Calibrator
- product5322A Electrical Safety Tester Calibrator
- product9500B Oscilloscope Calibrator
- product6135A/PMUCAL Phasor Measurement Unit Calibration System
- product6105A, 6100B Electrical Power Quality Calibrator
- product5730A High Performance Multifunction Calibrator
- product5522A Multi-Product Calibrator
- product5502A Multi-Product Calibrator
- Temperature Calibration
- Utility Locating Equipment
- Cable Avoidance Tools
- GPR
- Pipeline integrity and corrosion control
- Precision Locator Range
- productRadiodetection RD8200SG Survey Grade Locator
- productRadiodedection RD Map
- productRadiodetection RD7100 Cable, Pipe and RF Marker Locators
- productRadiodetection RD8100 Cable, Pipe and RF Marker Locators
- productRadiodetection RD7200 Cable and Pipe Locator
- productRadiodetection RD8200 Cable and Pipe Locators
- productRadiodetection Multifunction Signal Transmitter
- Handheld Thermal Cameras
- productFLIR T620
- productFLIR T640
- productFLIR T660
- productFLIR TG297 – Industrial High Temp Thermal Camera
- productFLIR TG165-X – MSX Thermal Camera
- productFLIR T865 – High-Performance Handheld Infrared Camera
- productFLIR T840 – High-Performance Thermal Camera with Viewfinder
- productFLIR T560 – Professional Thermal Camera
- productFLIR T540 – Professional Thermal Camera
- productFLIR T530 – Professional Thermal Camera
- productFLIR T1020 – HD Thermal Camera with Viewfinder
- productFLIR T1010 – HD Thermal Imaging Camera
- productFLIR One Pro LT – Pro-Grade Thermal Camera for Smartphones
- productFLIR ONE Pro – Pro-Grade Thermal Camera for Smartphones
- productFLIR E96 – Advanced Thermal Imaging Camera
- productFLIR E86 – Advanced Thermal Imaging Camera
- productFLIR E8-XT – Infrared Camera with Extended Temperature Range
- productFLIR E76 – Advanced Thermal Imaging Camera
- productFLIR E6-XT – Infrared Camera with Extended Temperature Range
- productFLIR E54 – Advanced Thermal Imaging Camera
- productFLIR E5-XT – Infrared Camera with Extended Temperature Range
- productFLIR E4 Wi-Fi – Infrared Camera with MSX Wi-Fi
- productFLIR E4 – Infrared Camera with MSX
- productFLIR C5 – Compact Thermal Camera
- productFLIR C3-X – Compact Thermal Camera
- Portable Gas Detectors
- Gas Detection Cameras
- Biomedical Equipment
- Condition monitoring
- Laboratory equipment for food and agriculture
- Moisture testing & Grain Analysis
- Grain Analysis
- Moisture testing
- productDICKEY-john mini GAC® plus
- productDICKEY-john mini GAC®
- productDICKEY-john Mini GAC® 2500
- productDICKEY-john GAC® 500 XT
- productDICKEY-john GAC® 2500-INTL Grain Analysis Computer
- productDICKEY-john GAC® 2100b
- productDICKEY-john GAC® 2100 GI
- productDICKEY-john GAC® 2100 Agri
- productDICKEY-john GAC ® 2100bsa
- Refractometer
- Abbe refractometer
- Abbe refractometer DR-A1/NAR series
- productAtago Abbe Refractometer NAR-2T・UH (for high temperature and high refractive index measurement)
- productAtago Abbe Refractometer NAR-2T・HI (for high temperature and high refractive index measurement)
- productAtago Abbe Refractometer NAR-2T・LO (for high temperature and low refractive index measurement)
- productAtago Abbe Refractometer NAR-1T・LO (for low refractive index measurement)
- productAtago Abbe Refractometer NAR-4T
- productAtago Abbe Refractometer NAR-3T
- productAtago Abbe Refractometer NAR-2T
- productAtago Abbe Refractometer NAR-1T SOLID
- productAtago Abbe Refractometer NAR-1T LIQUID
- productAtago Abbe Refractometer DR-A1-Plus
- Multi-wavelength Abbe Refractometer DR-M series
- Abbe refractometer DR-A1/NAR series
- Hybrid
- Brix and Salt Hybrid Meter PAL-BX|SALT
- Digital Differential Refractometer DD-7
- Digital Refractometer RX-i series
- Digital Refractometer RX-α series
- Pocket Brix-Acidity Meter PAL-BX|ACID
- productAtago Pocket Brix-Acidity Meter Multi Fruits PAL-BX|ACID F5 Master Kit
- productAtago Pocket Brix-Acidity Meter (Yogurt) PAL-BX|ACID96 Master Kit
- productAtago Pocket Brix-Acidity Meter (Milk) PAL-BX|ACID91 Master Kit
- productAtago Pocket Brix-Acidity Meter (Sake) PAL-BX|ACID121 Master Kit
- productAtago Pocket Brix-Acidity Meter (Beer) PAL-BX|ACID101 Master Kit
- productAtago Pocket Brix-Acidity Meter (Vinegar) PAL-BX|ACID181 Master Kit
- productAtago Pocket Brix-Acidity Meter (Coffee cherry) PAL-BX|ACID40 Master Kit
- productAtago Pocket Brix-Acidity Meter (Cherry) PAL-BX|ACID16 Master Kit
- productAtago Pocket Brix-Acidity Meter (Mango) PAL-BX|ACID15 Master Kit
- productAtago Pocket Brix-Acidity Meter (Pear) PAL-BX|ACID14 Master Kit
- productAtago Pocket Brix-Acidity Meter (Asian Pear) PAL-BX|ACID12 Master Kit
- productAtago Pocket Brix-Acidity Meter (Plum) PAL-BX|ACID11 Master Kit
- productAtago Pocket Brix-Acidity Meter (Pineapple) PAL-BX|ACID9 Master Kit
- productAtago Pocket Brix-Acidity Meter (Kiwi) PAL-BX|ACID8 Master Kit
- productAtago Pocket Brix-Acidity Meter (Blueberry) PAL-BX|ACID7 Master Kit
- productAtago Pocket Brix-Acidity Meter (Banana) PAL-BX|ACID6 Master Kit
- productAtago Pocket Brix-Acidity Meter (Apple) PAL-BX|ACID5 Master Kit
- productAtago Pocket Brix-Acidity Meter (Strawberry) PAL-BX|ACID4 Master Kit
- productAtago Pocket Brix-Acidity Meter (Tomato) PAL-BX|ACID3 Master Kit
- productAtago Pocket Brix-Acidity Meter (Grape & Wine) PAL-BX|ACID2 Master Kit
- productAtago Pocket Brix-Acidity Meter (Citrus) PAL-BX|ACID1 Master Kit
- Measure easily on-site
- Digital Refractometer Palette series
- productAtago Digital Wine Refractometer WM-7
- productAtago Digital Urine S. G. Refractometer UG-α
- productAtago Digital Butyro Refractometer PR-BUTYRO
- productAtago Digital Refractometer for Dimethylformamide PR-40DMF
- productAtago Digital Refractometer for Salinity PR-100SA
- productAtago Digital Refractometer for Ethyl Alcohol PET-109
- productAtago Digital Refractometer for a Water Solution of Hydrogen Peroxide PR-50HO
- productAtago Digital Refractometer for Isopropyl alcohol PR-60PA
- productAtago Digital Refractometer PR-RI
- productAtago Digital Refractometer PR-301α
- productAtago Digital Refractometer PR-201α
- productAtago Digital Refractometer PR-101α
- productAtago Digital Refractometer PR-32α (alpha)
- Digital Suction-Type Refractometer
- Hand-Held Refractometer MASTER™series
- productAtago Hand-Held Refractometer Veterinary MASTER-VET
- productAtago Clinical Refractometer MASTER-SUR/NM
- productAtago Clinical Refractometer MASTER-SUR/Nα
- productAtago Urine S. G. Refractometer MASTER-URC/NM
- productAtago Urine S. G. Refractometer MASTER-URC/Nα
- productAtago Battery Coolant Checker MASTER-BC
- productAtago Coolant Refractometer MASTER-BR
- productAtago Soy milk Refractometer MASTER-Soy MilkM
- productAtago Soy Milk Refractometer MASTER-Soy Milkα
- productAtago Ramen Soup Refractometer MASTER-Ramen M
- productAtago Ramen Soup Refractometer MASTER-Ramen α
- productAtago Honey Refractometer MASTER-HONEY/BX
- productAtago Salinity Refractometer MASTER-S28M
- productAtago Salinity Refractometer MASTER-S28α
- productAtago Salinity Refractometer MASTER-S10M
- productAtago Salinity Refractometer MASTER-S10α
- productAtago Brix & Salinity Refractometer MASTER-BX/S28M
- productAtago Salinity Refractometer MASTER-S/MillM
- productAtago Salinity Refractometer MASTER-S/Millα
- productAtago Hand Held Refractometer for Vegetable & Fruit MASTER-AGRI
- productAtago Hand Held Refractometer MASTER-RI
- productAtago Hand Held Refractometer MASTER-100H
- productAtago Hand Held Refractometer MASTER-93H
- productAtago Hand Held Refractometer MASTER-80H
- productAtago Hand Held Refractometer MASTER-50H
- productAtago Hand Held Refractometer MASTER-500
- productAtago Hand Held Refractometer MASTER-10PM
- productAtago Hand Held Refractometer MASTER-10PT
- productAtago Hand Held Refractometer MASTER-10Pα
- productAtago Hand Held Refractometer MASTER-10M
- productAtago Hand Held Refractometer MASTER-10T
- productAtago Hand Held Refractometer MASTER-10α
- productAtago Hand Held Refractometer MASTER-20PM
- productAtago Hand Held Refractometer MASTER-20PT
- productAtago Hand Held Refractometer MASTER-20Pα
- productAtago Hand Held Refractometer MASTER-20M
- productAtago Hand Held Refractometer MASTER-20T
- productAtago Hand Held Refractometer MASTER-20α
- productAtago Refractometer MASTER-PM
- productAtago Refractometer MASTER-PT
- productAtago Refractometer MASTER-Pα
- productAtago Refractometer MASTER-M
- productAtago Refractometer MASTER-T
- productAtago Refractometer MASTER-α
- productAtago Hand Held Refractometer MASTER-53PM
- productAtago Hand Held Refractometer MASTER-53PT
- productAtago Hand Held Refractometer MASTER-53Pα
- productAtago Hand Held Refractometer for Milky Samples MASTER-53S
- productAtago Hand Held Refractometer MASTER-53M
- productAtago Hand Held Refractometer MASTER-53T
- productAtago Hand Held Refractometer MASTER-53α
- PEN series
- productAtago Digital Hand-Held Urine Specific Gravity PEN-Urine S. G.
- productAtago Digital Dip-type Dried Fruit Moisture Meter PEN-Dried Fruit Moisture
- productAtago Digital Hand-held PEN-Ethanol W
- productAtago Digital Hand-held PEN-Ethanol V
- productAtago Digital Hand-held “PEN” Sodium chloride Refractometer PEN-SW (Baume)
- productAtago Digital Hand-held PEN-SW WV
- productAtago Digital Hand-held PEN-SW W
- productAtago Digital Hand-held PEN-PRO
- Pocket Refractometer PAL™Series
- productAtago Soy Milk Refractometer PAL-27S
- productAtago Ramen Soup Refractometer PAL-96S
- productAtago Salinity Refractometer PAL-03S
- productAtago Honey Refractometer PAL-22S
- productAtago Digital Hand-held PAL-Pâtissier
- productAtago Digital Pocket Refractometer PAL-COFFEE(BX)
- productAtago Peracetic acid Refractometer PAL-Peracetic acid
- productAtago Hypochlorous acid Refractometer PAL-Hypochlorous acid
- productAtago Ethyl alcohol Refractometer PAL-COVID-19
- productAtago Digital Hand-held”Pocket” Refractometer PAL-SDGs
- productAtago Digital Hand-held PAL-LOOP
- productAtago Digital Hand-held PAL-2
- productAtago Digital Hand-held PAL-RI
- productAtago Digital Hand-held PAL-BX/RI
- productAtago Digital Hand-held PAL-S
- productAtago Digital Hand-held “Pocket” Refractometer PAL-H
- productAtago Digital Hand-held PAL-α
- productAtago Digital Hand-held PAL-1
- productAtago Digital Hand-held PAL-3
- Digital Refractometer Palette series
- Abbe refractometer
- Feed and Cereals Analysis
- Honey Analysis
- Meat and Seafood Analysis
- Milk analysis
- Moisture testing & Grain Analysis
- Animal health (Vaccine)
- Cable Equipments
- Company
- Calibration
- Service
- Training
- News
- Contact
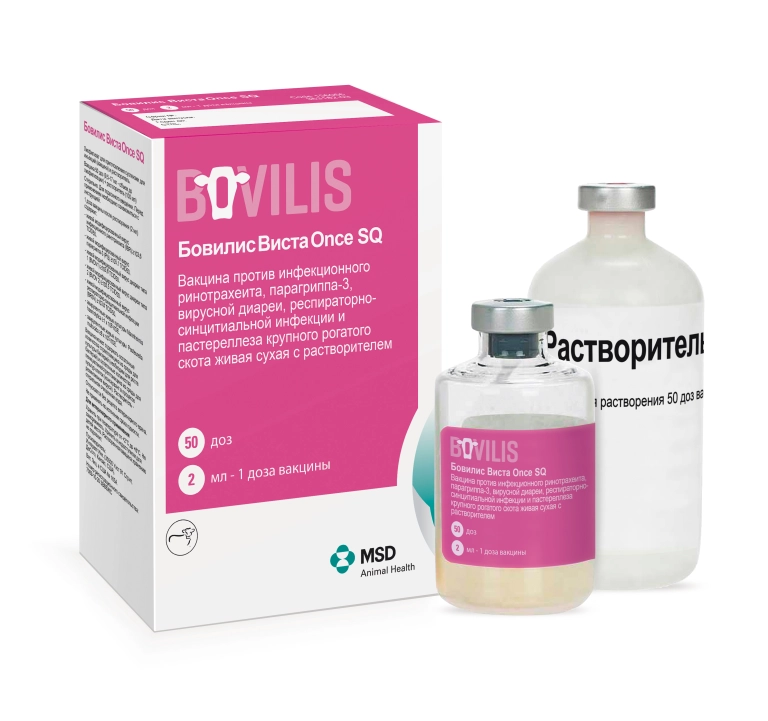
Bovilis Vista Once SQ (Бовилис Виста Once SQ )
Vaccine against infectious rhinotracheitis, parainfluenza-3, viral diarrhea, respiratory syncytial infection and pasteurellosis in cattle live dry with a solvent.
Composition One immunizing dose of the vaccine after dissolution (2 ml) contains:
- live modified infectious rhinotracheitis virus (IBR) ≥103.6 TCID50,
- live modified parainfluenza-3 virus (PI3) ≥105.1 TCID50,
- live modified diarrhea virus type 1 (BVDV1) ≥103.8TCID50,
- live modified diarrhea virus type 2 (BVDV 2) ≥103.5 TCID50,
- live modified respiratory syncytial virus (BRSV) ≥103.8 TCID50,
- avirulent live cultures of Mannheimia haemolytica ≥1 x 106 CFU,
- avirulent live cultures of Pasteurella multocida ≥8 x 105 CFU.
- Dosage form Lyophilisate for suspension for injection (vaccine) and solvent.
- Indications for use For active immunization of cattle to prevent diseases caused by infectious rhinotracheitis virus, bovine diarrhea virus (types 1 and 2), respiratory syncytial infection virus, parainfluenza-3 virus, Mannheimia haemolytica and Pasteurella multocida. Also, the vaccine is used in cows and heifers before insemination to prevent infection of the fetus and reduce the number of abortions due to exposure to diarrhea viruses (type 1 and 2) and infectious rhinotracheitis.
